Group 6/16 - The Chalcogens
The chalcogens (/ˈkælkədʒɪnz/) are the chemical elements in group 16 of the periodic table. This group is also known as the Oxygen family. It consists of the elements Oxygen (O), Sulphur (S), Selenium (Se), Tellurium (Te), and the radioactive element Polonium (Po). The chemically uncharacterized synthetic element Livermorium (Lv) is predicted to be a chalcogen as well. Often, Oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from Sulfur, Selenium, Tellurium, and Polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning Copper (the term was also used for bronze/brass, any metal in the poetic sense, ore or coin), and the Latinised Greek word genēs, meaning born or produced.
|
|
|
|
|
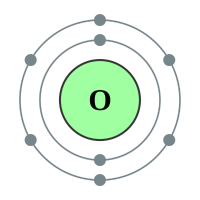
Oxygen |
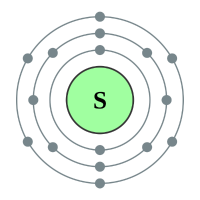
Sulphur (Sulfur) |
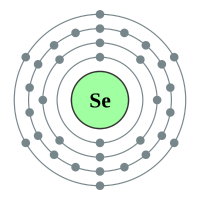
Selenium |
|
2 shells - 8 electrons - [2,6] |
3 shells - 16 electrons - [2,8,6] |
4 shells - 34 electrons - [2,8,18,6) |
|
|
|
|
|
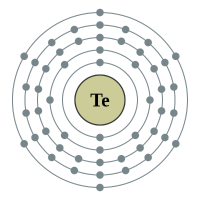
Tellurium |
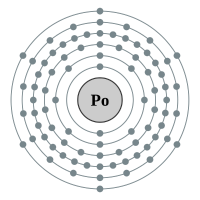
Polonium |
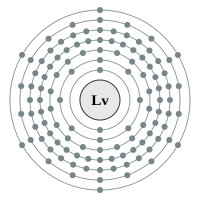
Livermorium |
|
5 shells - 52 electrons - [2,8,18,18,6] |
6 shells - 83 electrons - [2,8,18,32,18,6] |
7 shells - 116 electrons - [2,8,18,32,32,18,6] |
Sulphur has been known since antiquity, and Oxygen was recognized as an element in the 18th century. Selenium, Tellurium and Polonium were discovered in the 19th century, and Livermorium in 2000. All of the chalcogens have six valence electrons, leaving them two electrons short of a full outer shell. Their most common oxidation states are −2, +2, +4, and +6. They have relatively low atomic radii, especially the lighter ones.
Lighter chalcogens are typically nontoxic in their elemental form, and are often critical to life, while the heavier chalcogens are typically toxic. All of the naturally occurring chalcogens have some role in biological functions, either as a nutrient or a toxin. Selenium is an important nutrient (among others as a building block of Selenocysteine) but is also commonly toxic. Tellurium often has unpleasant effects (although some organisms can use it), and Polonium (especially the isotope Polonium-210) is always harmful as a result of its radioactivity.
Sulphur has more than 20 allotropes, Oxygen has nine, Selenium has at least five, Polonium has two, and only one crystal structure of Tellurium has so far been discovered. There are numerous organic chalcogen compounds. Not counting Oxygen, organic Sulphur compounds are generally the most common, followed by organic Selenium compounds and organic Tellurium compounds. This trend also occurs with chalcogen pnictides and compounds containing chalcogens and carbon group elements.
Oxygen is generally obtained by separation of air into Nitrogen and Oxygen. Sulphur is extracted from oil and natural gas. Selenium and Tellurium are produced as byproducts of Copper refining. Polonium and Livermorium are most available in particle accelerators. The primary use of elemental Oxygen is in steelmaking. Sulphur is mostly converted into Sulphuric acid, which is heavily used in the chemical industry.[6] Selenium's most common application is glassmaking. Tellurium compounds are mostly used in optical disks, electronic devices, and solar cells. Some of Polonium's applications are due to its radioactivity.
Back To >> [A] Oxidation State <<